Product Portfolio Mastered
Our Coverage, Your Edge
Innovator Pharmaceuticals
Generics Pharmaceuticals
Combination Products
Vaccines and Parentals
Biologics and Biosimilars
Cell and Gene Therapy
APIs
Homeopathic Drugs
Immunotherapy
OTC Drugs
Herbal Medicines
Veterinary Products
Global Regulators Mastered
Our Coverage, Your Advantage

- National and regional health agencies across 200+ countries, supporting navigation of Global Regulatory Frameworks.
- Updates from 1500+ Health authorities ranging from policies and regulations to news and important HA Communications (product recalls, warning letters, meeting summaries)


- Constant coverage across 200+ regulatory groups such as ICH, WHO, ASEAN, MERCOSUR, PAHO, GCC, PIC/S, etc. to strengthen International Regulatory Compliance.
- Regulatory recommendations, binding and non-binding regulations

- British, United States, European, Indian, Japanese, Argentine, Brazilian, Croatian, French and Korean


- Track of updates from 220+ prominent trade associations across the world such as PhRMA, PDA, EFPIA, JPMA and many more
- Position papers, government submissions, white papers and scientific publications on variety of topics
Bridging Regulatory Gaps with
AI-Powered Intelligence
freya.intelligence – our AI-powered Regulatory Intelligence Software

SMEs get repetitive “what’s required in X market for Y product?” across GxP areas.
An AI-powered, multilingual chatbot trained on curated pharma regulations delivering precise, cited, context answers in seconds for effective Regulatory Compliance Monitoring.


Evolving global rules disrupt clinical, CMC, labelling, and safety plans.
Timely, actionable alerts with custom delivery settings to be on top of changing updates and to stay submission-ready.


Unstructured, inconsistent requirements slow the retrieval of CTD specifics and timelines.
A single, verified Regulatory Intelligence database across 200+ markets and 100,000+ regulations.


Dense ICH/FDA/EMA texts, SOPs, and reports delay interpretation and decisions.
Interact with guidances and dossiers in natural language, get cited summaries, comparisons, and translations you can defend.


Regulatory data overload and siloed sources limit pharma teams from exploring cross-country and thematic requirements quickly.
Intuitive, modular, AI-powered dashboards with configurable, interactive visualizations that turn complex datasets into clear, actionable analytics


Health Authority queries (information requests, deficiency letters) stretch timelines and scatter knowledge.
Auto-ingest and classify queries, surface prior responses, draft replies based on past-precedents, and track deadlines to accelerate outcomes.

Your All-In-One Regulatory Solutions
freya.intelligence in Action
Use Cases
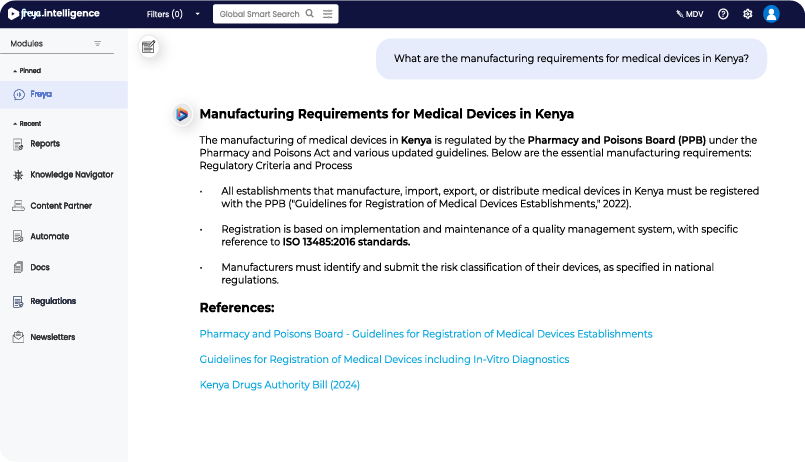
Simplifying EMA Biosimilar MAA Requirements
- Ask freya for EMA-specific biosimilar MAA requirements and references.
- Instantly receive structured, expert-verified guidance for faster submission planning.
Quick Access to Latest Vaccine Regulations in China
- Use freya.regulations to instantly filter NMPA updates by market, product type, and date.
- Get verified documents with summaries, source links, and safety or packaging insights in seconds to support ongoing Pharma Compliance

Tailored Regulatory Alerts for Global Product Portfolios
- Configure freya.alerts for weekly, customized updates by market, product type, or compliance area.
- Receive concise, expert-verified summaries with AI highlights for proactive monitoring.

Visualizing Global Regulatory Trends for Strategic Decisions
- Use freya.dashboards for real-time heatmaps, timelines, and trend analytics across 1,800+ authorities.
- Drive data-backed strategy and regulatory foresight through visual intelligence.

Streamlined Health Authority Query (RTQ) Management
- Manage all HA queries in one platform with freya.RTQ and AI-assisted drafting.
- Automate reviews, track issues, and speed up response cycles through smart workflows.

FAQs
Medicinal Products Regulatory Intelligence involves monitoring and interpreting global pharmaceutical regulatory standards that impact drug development and approvals. It helps pharma teams stay compliant from early development to market launch through pharmaceutical regulatory intelligence.
A Global Regulatory Intelligence Platform for medicinal products centralizes updates from across the world into a single regulatory intelligence database. It helps teams analyze this information to deliver actionable international regulatory compliance insights, all backed by expert-verified guidance.
Regulatory Intelligence enables accurate interpretation of global regulatory frameworks, reducing delays and compliance risks. It strengthens planning and execution across regulatory submission processes.
The platform continuously tracks regulatory information from multiple authorities and regions to maintain compliance. It supports proactive monitoring, gap identification, automated alerts and market-specific requirement mapping.
Regulatory Intelligence supports lifecycle management from development to post-approval changes. It helps you in staying up-to-date and ensuring alignment with evolving global pharmaceutical regulations at every stage.
It centralizes global regulatory tracking, enabling faster identification of authority updates. This simplifies pharmaceutical legislation tracking and supports proactive compliance planning.
AI-powered regulatory intelligence accelerates analysis and comparison of global regulations while improving regulatory compliance monitoring. It reduces manual effort through scalable, automated regulatory insights. freya.intelligence adds further value with multilingual AI responses and expert-verified regulatory data.