Product Portfolio Mastered
Our Coverage, Your Edge
In Vitro Diagnostics (IVD)
Software as a Medical Device (SaMD)
Implantable Devices
AI/ML Enabled Devices
Digital Health Solutions
Diagnostic Imaging Systems
Surgical and Orthopedic Devices
Cardiovascular and Respiratory Devices
Dental and Orthodontic Devices
Ophthalmic Devices
Urology and Gynaecology Devices
Radiology and Oncology Devices
Global Regulators Mastered
Our Coverage, Your Advantage

- National and regional health agencies across 200+ countries
- Updates from 1500+ Health Authorities covering device regulations, vigilance alerts, recalls, and policy updates


- Continuous coverage across 200+ global bodies such as IMDRF, WHO, ASEAN, MERCOSUR, PAHO, and GCC ensuring alignment with International Medical Device Regulatory Frameworks.
- Regulatory recommendations, harmonized guidance, and non-binding frameworks

- Monitoring updates from ISO, IEC, ASTM and key pharmacopoeias like US, EU, Japanese, and Indian
- Standards on QMS, clinical evaluation, safety, and performance testing


- Tracking updates from 220+ global trade bodies such as MedTech Europe, AdvaMed, APACMed, and MDMA
- Position papers, policy submissions, and white papers across diverse device categories
Bridging Regulatory Gaps with
AI-Powered Intelligence

RA teams face repetitive questions like “What’s required in X market for Y device type?” across MDR, IVDR, and regional frameworks.
An AI-powered, multilingual chatbot trained on curated device regulations, delivering precise, referenced answers across global markets in seconds.


Frequent MDR/IVDR revisions, IMDRF updates, and local HA notices disrupt submissions and planning.
Timely, actionable alerts with custom delivery settings to track regulatory changes and stay audit- and submission-ready through robust Compliance Monitoring for Medical Devices.


Unstructured, inconsistent device classifications and documentation templates hinder efficient regulatory referencing.
A single, verified Regulatory Intelligence Database of global medical device and IVD regulations covering 200+ markets and 100,000+ entries.


Dense MDR annexes, guidance documents, and standards slow interpretation and response timelines.
Interact with regulatory texts in natural language; get instant summaries, comparisons, and translations you can rely on.


Change control, technical documentation updates, and vigilance actions are scattered across systems.
Configurable, auditable workflows aligned with ISO 13485 and MDR QMS principles, featuring assignments, SLAs, impact assessments, and attachments.


Health Authority queries (deficiency letters, safety clarifications) delay responses and fragment institutional knowledge.
Auto-ingest, classify, and retrieve historic responses, draft replies using past precedents and track all timelines centrally.


Health Authority queries (deficiency letters, safety clarifications) delay responses and fragment institutional knowledge.
Auto-ingest, classify, and retrieve historic responses, draft replies using past precedents and track all timelines centrally.

Your All-In-One Regulatory Solutions
Freya.Intelligence in Action
Use Cases
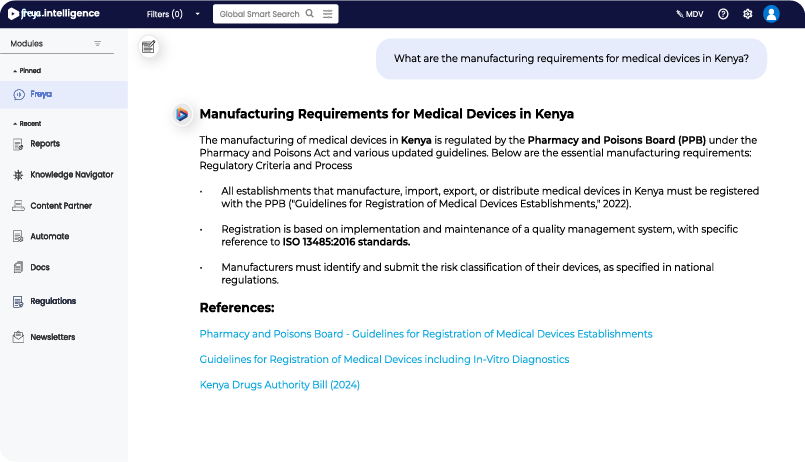
FDA Guidance Access for SaMD Submission Readiness
- Use freya.regulations to instantly filter recent FDA updates for Software as a Medical Device.
- Retrieve targeted documents on cybersecurity, clinical validation, and software functions to align faster.
Custom Alerts for Global Medical Device Compliance
- Configure freya.alerts for weekly, tailored updates across US, EU, China, and Japan.
- Get concise, source-linked summaries on UDI, labeling, and clinical evaluation to act proactively.

Unified Standards Monitoring for Global Device Compliance
- Track ISO, IEC, ASTM, and national standards in one dashboard with smart filters and summaries.
- Stay current on sterilization, software, and risk management updates to keep QMS documentation compliant.

Efficient Query Management for MDR-Ready Device Teams
- Manage Notified Body and HA queries with freya.RTQ using AI-assisted search and workflows.
- Standardize responses, cut turnaround time, and pre-empt common MDR issues through tracked insights.

Streamlined Health Authority Query (RTQ) Management
- Manage all HA queries in one platform with freya.RTQ and AI-assisted drafting.
- Automate reviews, track issues, and speed up response cycles through smart workflows.

FAQs
Medical Device Regulatory Intelligence refers to the continuous monitoring, analysis, and interpretation of global requirements impacting device classification, approval, and lifecycle management. It helps organizations navigate international medical device regulatory frameworks and ensures alignment with evolving standards. This forms a strong foundation for maintaining global compliance.
Regulatory Intelligence consolidates medical device regulatory information across multiple authorities, enabling faster understanding of market-specific requirements. It ensures teams stay compliant with rapid medical device regulatory updates from FDA, EU MDR, NMPA and other bodies. freya.intelligence strengthens this through structured insights and automated monitoring across 200+ markets.
It helps companies stay ahead of constant medical device regulatory updates, reducing delays and compliance risks. Regulatory Intelligence supports accurate interpretation of global rules, improving readiness for audits and submissions. This is essential for predictable and efficient approval pathways.
A Regulatory Intelligence Platform for Medical Devices centralizes worldwide guidelines, classifications, and documentation needs in a single source from multiple authorities and regulatory bodies. It streamlines research time, accelerates decision-making, and enhances cross-functional coordination. freya.intelligence adds efficiency with AI-driven search, multilingual answers, and curated expert-verified content and regulatory data.
Real-time regulatory intelligence for medical devices ensures instant visibility into critical changes across global markets. It helps teams react quickly to new submissions requirements, standards revisions, and safety expectations. This reduces compliance gaps and strengthens global operational agility.
It provides timely insights into medical device risk management regulatory intelligence, helping companies align with global expectations. By tracking risk-related updates, alerts, and safety notices, it enables proactive mitigation planning. This results in more robust product-risk control throughout the device lifecycle.
Regulatory Intelligence helps organizations interpret evolving post-market obligations, recall notices, vigilance rules, and reporting criteria. Through continuous medical device regulatory updates, it strengthens surveillance systems and supports timely corrective actions. This enhances overall safety compliance and product performance visibility.